![]()
ACHIEVEMENTSIPR The University of Osaka
-

- ACHIEVEMENTS
- Biological lasso: Enhanced drug delivery to the brain
Press Release
2022.11.09
Biological lasso: Enhanced drug delivery to the brain
In a study recently published in the journal Nature Biomedical Engineering, researchers from Kanazawa University use a method called “lasso-grafting” to design therapeutics with enhanced longevity and brain penetration
Osaka, Japan: Cell growth and repair are stimulated by biomolecules known as cytokines and growth factors. Unfortunately, delivering adequate concentrations of these molecules to the brain for treating neurological conditions like Alzheimer’s disease is challenging as they are either cleared out of the blood very quickly or do not penetrate neural tissue effectively. A research team led by Junichi Takagi at Osaka University in collaboration with Kunio Matsumoto and Katsuya Sakai at Kanazawa University and Hiroaki Suga, the University of Tokyo has now used a technique called “lasso-grafting” to design molecules that replicate growth factors with longer retention in the body and brain penetration.
The team synthesized a molecular entity comprising two components: macrocyclic peptides inserted into antibody fragments (known as Fc) (Fig. 1a). Macrocyclic peptides are truncated proteins which can be engineered to resemble growth factors. Using lasso-grafting, a method previously developed by the researchers, the selected peptides were inserted into loops found on Fc. Now, lasso-grafting ensures that the macrocyclic peptides are easily exposed while keeping the structural integrity and function of both the peptide and Fc intact. Fc was used for this purpose as it remains in the body long enough and can easily added functionality of the Fab of choice.
Using this process, a designer molecule replicating the hepatocyte growth factor (HGF) was first created. HGF binds a docking protein known as Met on the surface of cells to initiate signaling for cell growth and survival. Thus, aMD4 and aMD5, two macrocyclic peptides that can also bind to Met were first identified. They were then grafted into various sites on Fc until optimum insertion sites were found. When exposed to cells, Fc(aMD4) and Fc(aMD5) indeed latched onto Met receptors and initiated cellular signaling akin to HGF (Fig. 1b).
Next, the longevity of Fc(aMD4) compared to Fc and HGF alone, was examined. When administered to mice, concentrations of HGF dwindled significantly after an hour while Fc(aMD4) persisted at levels enough to activate Met, for up to 200 hours. Markers for cellular replication were also active in these mice. Fc(aMD4) thus showed longevity and bioactivity. The final step was to determine the brain penetration of these designer molecules. For this purpose, aMD4 was inserted into an Fc of anti-transferrin receptor (TfR) antibody which accumulates in the mouse brain after peripheral administration (Fig. 1c). Indeed, TfR(aMD4) showed high accumulation and retention within the brain tissues of mice compared to Fc(aMD4) alone.
This study depicts a novel strategy of inducing the effects of growth factors and cytokines with enhanced retention in brain tissues. What’s more, based on the macrocyclic peptides and antibodies selected, this technique can be applied to imitate several growth factors. “Thus, lasso-grafting enables the design of protein therapeutics with the desired physicochemical stability and controllable pharmacokinetics, as well as the rapid engineering of antibodies for multiple functionalities,” suggest the researchers.
Background
Macrocyclic peptides: Macrocyclic peptides are short fragments of proteins. Their cyclic structure facilitates strong binding to proteins of interest. Macrocyclic peptides are under investigation in drug development because advanced techniques as in vitro displays now allow for easy discovery of peptides that can bind to a desired target. The lasso-grafting method developed by the researchers here also allows for easy incorporation of macrocyclic peptides into protein scaffolds which can generate multifunctional proteins.
Related Image
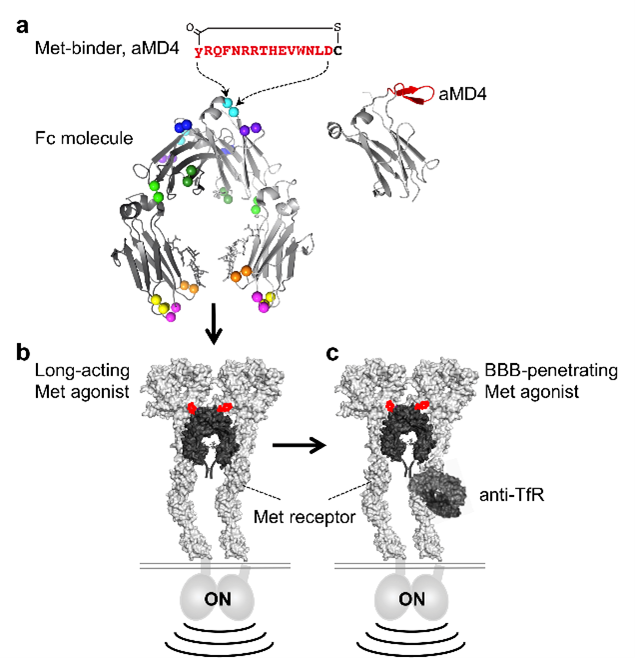
Fig. 1. Lasso-grafting to design molecules that mimic growth factors with longer retention and brain penetration. (a) A pharmacophore sequence of Met receptor-binding macrocyclic peptide (aMD4; shown in red) was inserted into the loops (coloured balls) of human IgG1 Fc protein. (b) Lasso-grafting Fc yields Met agonists with extended half-life in the body. (c) Lasso-grafting Fc of an anti-TfR antibody yields Met agonists with blood-brain-barrier (BBB) penetrance.
© 2022 Sakai, et al.
Title: Designing receptor agonists with enhanced pharmacokinetics by grafting macrocyclic peptides into fragment crystallizable regions
Journal: Nature Biomedical Engineering
Authors: Katsuya Sakai, Nozomi Sugano-Nakamura, Emiko Mihara, Rojas-Chaverra NM, Sayako Watanabe, Hiroki Sato, Ryu Imamura, Dominic Chih-Cheng Voon, Itsuki Sakai, Chihiro Yamasaki, Chise Tateno, Mikihiro Shibata, Hiroaki Suga, Junichi Takagi, and Kunio Matsumoto
DOI: 10.1038/s41551-022-00955-6
Funded by:
Japan Society for the Promotion of Science
Japan Agency for Medical Research and Development
World Premier International Research Center Initiative (WPI), MEXT, Japan
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan’s leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan’s most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en