Beamline for Biological Macromolecular Assemblies at SPring-8 (BL44XU)
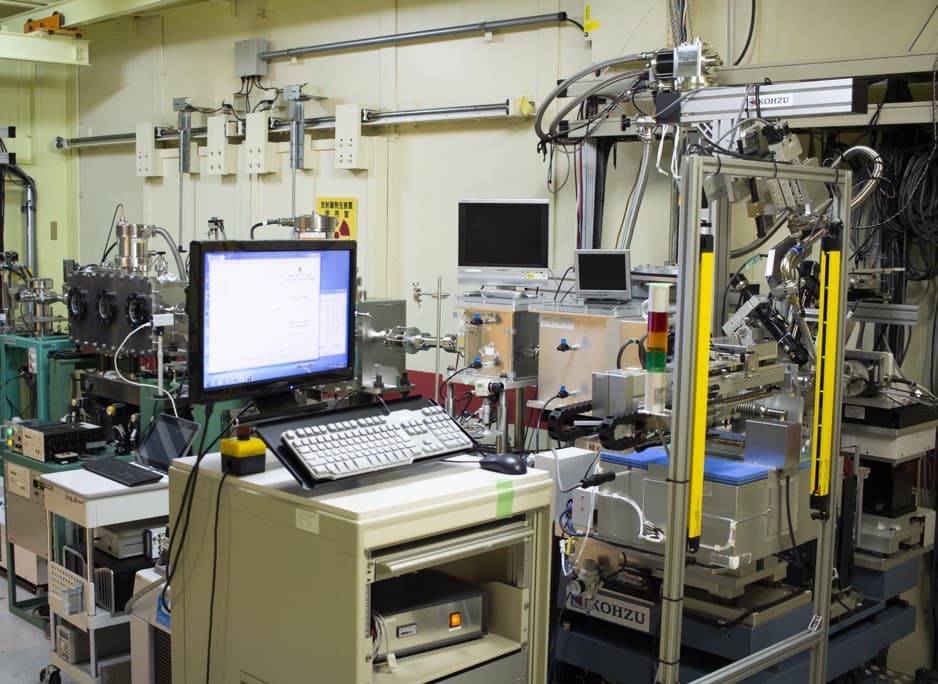
The beamline for X-ray crystallography of biological macromolecules at SPring-8 (BL44XU) is available to researchers who belong to academic organizations throughout the world. Regular applications involving non-proprietary proposals are received once per year, and urgent applications can be accepted all year around. More than 50% of the total beamtime is used for collaborative research programs of the Institute for Protein Research. This beamline is specially designed for data collection of large unit cell crystals for analyzing biological macromolecular assemblies, such as protein complexes, protein-nucleic acid complexes, and viruses.
|
Year established |
1999 (Upgraded in 2001, 2003, 2006, 2009, 2011, 2012, 2015, 2018) |
|---|---|
|
Facility specification |
Light source: |
Solution NMR Spectrometer in an Ultra-high Magnetic Field

The ultra high field NMR instruments ( ¹H resonance frequency of 950 and 800 MHz) enable the analysis of 3D structures dynamics and interactions of high molecular-weight protein complexes and organic molecules at low concentrations, which markedly exceeds the performance of conventional spectrometers. This device provides one of the world’s highest magnetic fields, following a 1200 MHz spectrometer.
600, 500, and 400 MHz NMR instruments are also available, allowing structure, dynamics, and interaction analysis of proteins and small molecules to be conducted.
|
Year established |
2010 (Upgraded in 2013, 2014 and 2020) |
|---|---|
|
Equipments |
950, 800, 600, 500, and 400 MHz NMR Spectroscopy (BRUKER) * |
Ultra-High-Sensitivity Solid-State NMR Spectrometers

These spectrometers enable the analysis of the structures and functions of biomolecules in non-crystalline solid states, such as proteins in lipid bilayers and amyloid proteins. The two DNP-NMR spectrometers are equipped with gyrotrons generating high-power submillimeter waves and sample-rotation systems at cryogenic temperatures. Thereby, they generate hyper-polarization that provide the world’s highest performance in NMR sensitivity.
|
Year established |
2001 (Upgraded in 2006 and 2013) |
|---|---|
|
Equipment |
500 MHz Solid-State NMR Spectrometer |

IPR is equipped with cutting-edge cryo-electron microscopy instruments for the precise structural analysis of biological samples. In addition to the single-particle analysis for purified biomolecules, we cover all aspects of electron structural studies, including electron tomography for biomolecules in cells as well as microED for small molecules. IPR offers a complete range of equipment for sample preparation, such as a high-pressure freezing, a cryo-microtome, and a blot-free cryo-grid preparation robot, to meet diverse observation needs.
|
Year extablished |
2012 (Upgraded in 2015 and 2017) |
|---|---|
|
Equipments |
120kV H-7650(2010-) |
Protein sequencer (Shimadzu PPSQ-53A)

Amino acid sequence (primary structure) of a protein is an essential information for studying protein structure and function. Full-length amino acid sequences of proteins are easily obtained from the information of genome, however, proteins in the actual living system are sometimes truncated at N-terminus or/and C-terminus or modified by post-translational modification.
Therefore, chemical sequencing using Edman degradation method gives valuable sequence information. In addition, the amino acid sequences of proteins of a species without genome information must be directly analyzed with the protein samples themselves.
Our protein sequencer, Shimadzu PPSQ-53A can analyze the N-terminal sequence of a protein or a peptide. We accept requests from outside IPR as commissioned analyses.
|
Year established |
2019 |
|---|---|
|
Equipment |
Shimadzu PPSQ-53A |